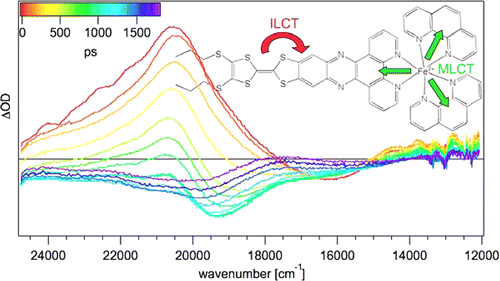
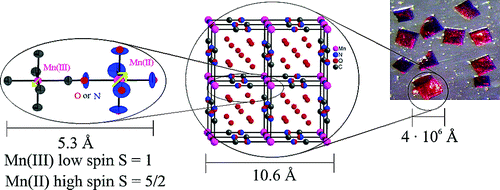
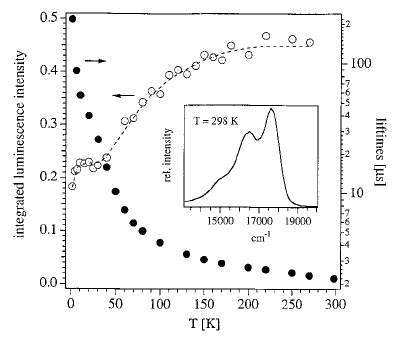
In analogy to the [MII(bpy)3]2+¬†cations, where MII¬†is a divalent transition-metal and bpy is 2,2‚Äė-bipyridine, the tris-chelated [MIII(bpy)3]3+¬†cations, where MIII¬†is CrIII¬†or CoIII, induce the crystallization of chiral, anionic three-dimensional (3D) coordination polymers of oxalate-bridged (őľ-ox) metal complexes with stoichiometries [MII2(ox)3]n2n-¬†or [MIMIII(ox)3]n2n-. The tripositive charge is partially compensated by inclusion of additional complex anions like ClO4-, BF4-, or PF6-¬†which are encapsulated in cubic shaped cavities formed by the bipyridine ligands of the cations. Thus, an elaborate structure of cationic and anionic species within a polymeric anionic network is realized. The compounds isolated and structurally characterized include [CrIII(bpy)3][ClO4] [NaCrIII(ox)3] (1), [CrIII(bpy)3][ClO4][MnII2(ox)3] (2), [CrIII(bpy)3][BF4] [MnII2(ox)3] (3), [CoIII(bpy)3][PF6][NaCrIII(ox)3] (4). Crystal data:¬†¬†1, cubic,¬†P213,¬†a¬†= 15.523(4) √Ö,¬†Z¬†= 4;¬†2, cubic,¬†P4132,¬†a¬†= 15.564(3) √Ö,¬†Z¬†= 4;¬†3, cubic,¬†P4132,¬†a¬†= 15.553(3) √Ö,¬†Z¬†= 4;¬†4, cubic,¬†P213,¬†a= 15.515(3) √Ö,¬†Z¬†= 4. Furthermore, it seemed likely that 1,2-dithiooxalate (dto) could act as an alternative to the oxalate bridging ligand, and as a result the compound [NiII(phen)3][NaCoIII(dto)3]¬∑C3H6O (5) has successfully been isolated and structurally characterized. Crystal data:¬†¬†5, orthorhombic,¬†P212121,¬†a¬†= 16.238(4) √Ö,¬†b¬†= 16.225(4) √Ö,¬†c¬†= 18.371(5) √Ö,¬†Z¬†= 4. In addition, the photophysical properties of compound¬†1¬†have been investigated in detail. In single crystal absorption spectra of [CrIII(bpy)3][ClO4][NaCrIII(ox)3] (1), the spin‚ąíflip transitions of both the [Cr(bpy)3]3+¬†and the [Cr(ox)3]3-¬†chromophores are observed and can be clearly distinguished. Irradiating into the spin-allowed¬†4A2¬†‚Üí¬†4T2absorption band of [Cr(ox)3]3-¬†results in intense luminescence from the¬†2E state of [Cr(bpy)3]3+as a result of rapid energy transfer processes. |